This website is best viewed using the horizontal display on your tablet device.
- INDICATIONS

ABRAXANE® is indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated.
ABRAXANE® is indicated for the first-line treatment of locally advanced or metastatic non–small cell lung cancer, in combination with carboplatin, in patients who are not candidates for curative surgery or radiation therapy.
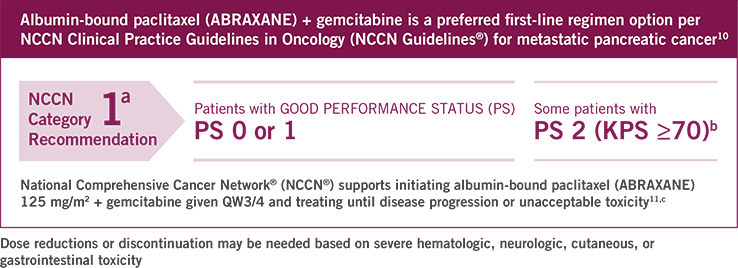
ABRAXANE® is indicated for the first-line treatment of patients with metastatic adenocarcinoma of the pancreas, in combination with gemcitabine.
- FULL PRESCRIBING INFORMATION INCLUDING BOXED WARNING
- PATIENT INFORMATION
- PATIENT SITES
- BMS ACCESS SUPPORT
- BMS RESOURCES
THIS SITE IS INTENDED FOR U.S. HEALTHCARE PROFESSIONALS ONLY.

This website is best viewed using the vertical display on your mobile device.
THIS SITE IS INTENDED FOR U.S HEALTHCARE PROFESSIONALS ONLY.
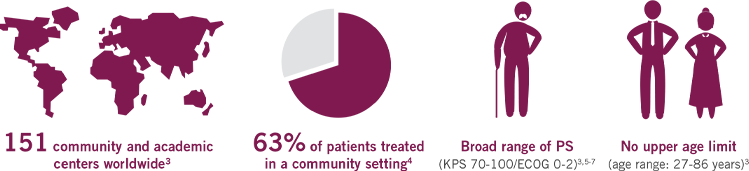
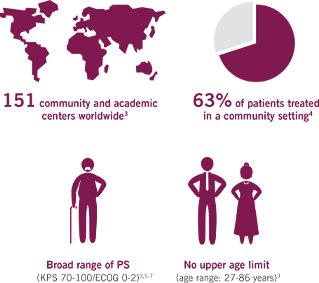
A TRIAL REFLECTIVE OF THE PATIENTS TYPICALLY SEEN IN YOUR PRACTICE
The first Phase III trial for 1L mPC based on a real-world population3
MPACT is the largest multinational trial (861 patients) in the mPC setting
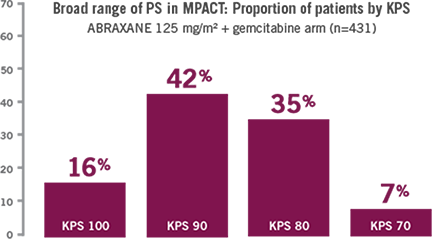
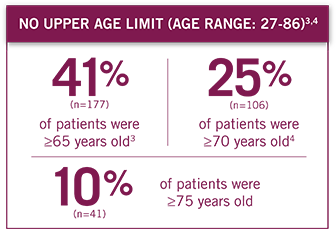
Study Design
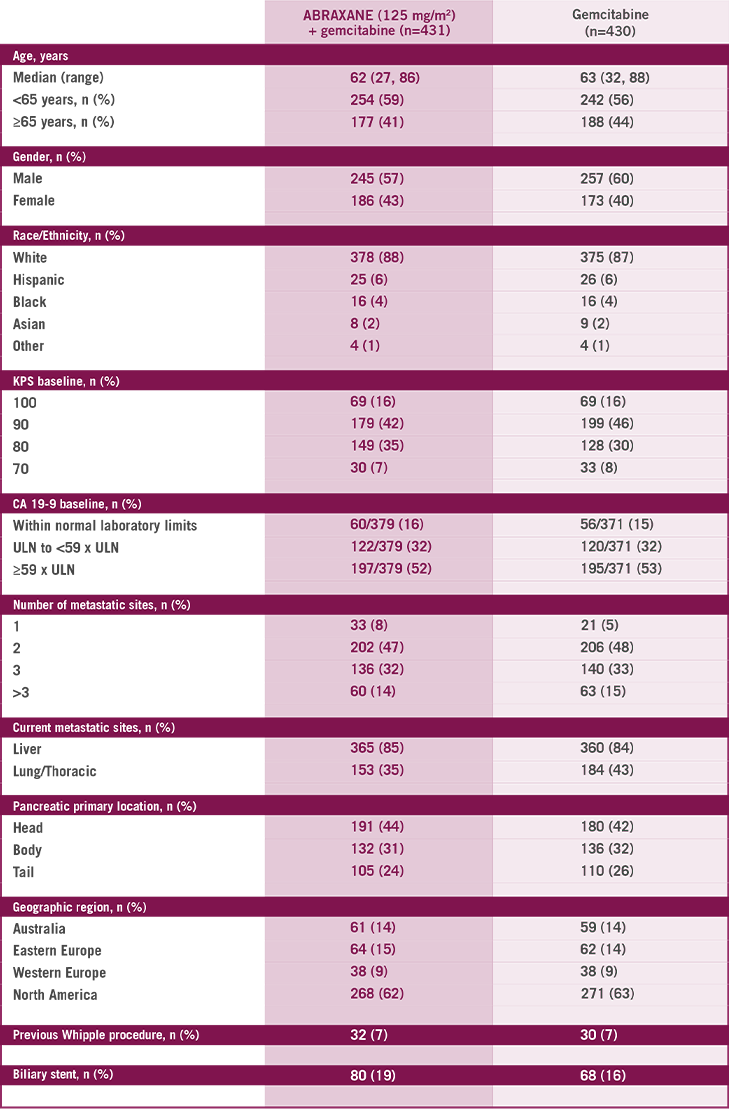
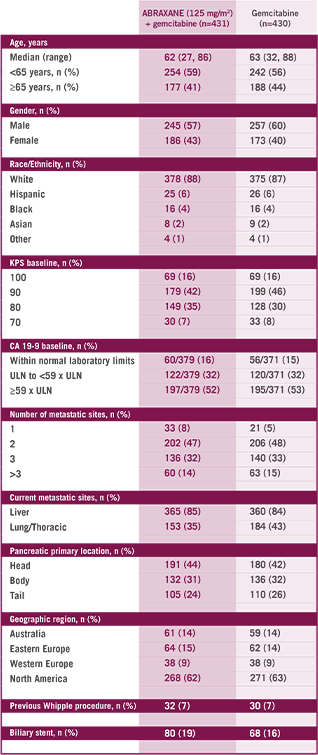
The multinational, randomized, Phase III MPACT study compared ABRAXANE + gemcitabine vs gemcitabine alone as first-line treatment in 861 patients with mPC with normal bilirubin and no prior chemotherapy in the metastatic setting. Primary endpoint was OS. ABRAXANE
1L=first-line; ECOG=Eastern Cooperative Oncology Group; KPS=Karnofsky Performance Status; MPACT=Metastatic Pancreatic Adenocarcinoma Clinical Trial; mPC=metastatic pancreatic cancer; OS=overall survival; PS=performance status; QW3/4=weekly for 3 of 4 weeks; QW7/8=weekly for 7 of 8 weeks.
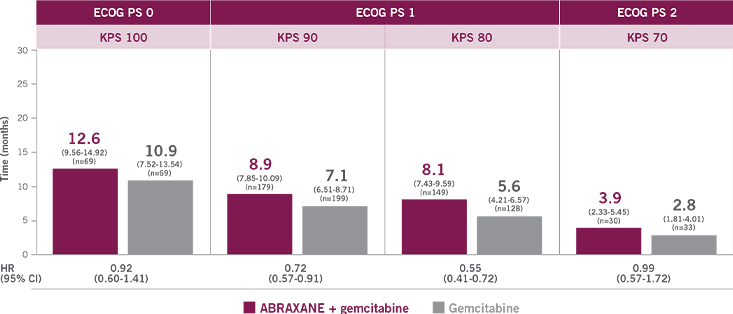
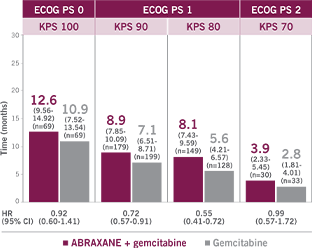
UNDERSTANDING THE RELATION BETWEEN
PERFORMANCE STATUS MEASUREMENTS5-7
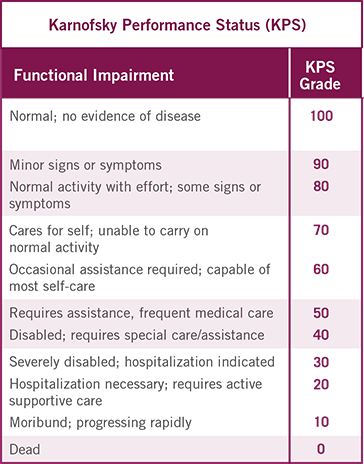

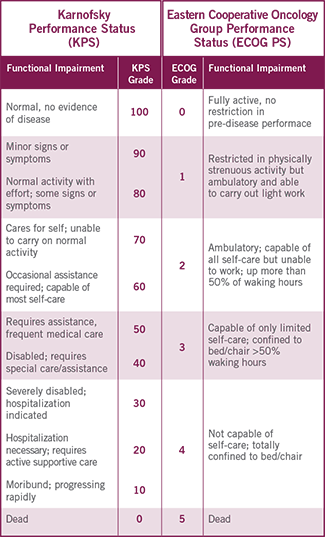
This proposed conversion scale was constructed empirically from a sample of patients (n=1385) with advanced cancer, including different tumor types. Both KPS and ECOG PS were determined by 7 physicians for each patient. This conversion scale had the highest rate of agreement (75%) observed among all possible scales.7
CLOSE THE TAB
WATCH
Dr. Kim Discusses the Largest Multinational Phase III Study

EFFICACY THAT DELIVERS
Only ABRAXANE + gemcitabine is proven to significantly improve OS across a broad range of patients3,a
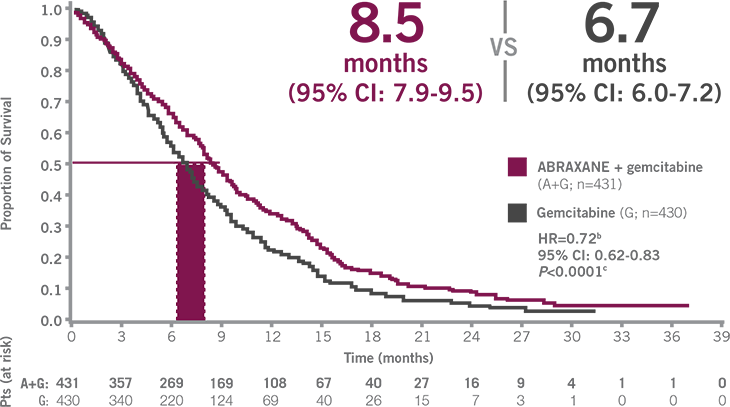
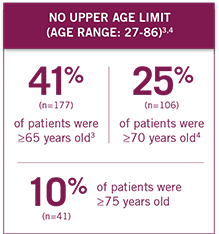
Patient performance status ranged from ECOG 0-2, with no upper age limit (age range: 27-86 years).3
Stratified using Cox proportional hazard model.
Based on a stratified log-rank test (stratified by geographic region, KPS, and presence of liver metastasis).
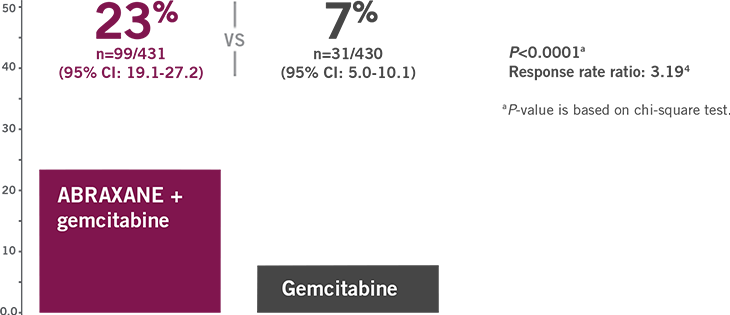
RESPONSE MATTERS:
ORR ASSESSED BY INDEPENDENT REVIEW
ABRAXANE (125 mg/m2 QW3/4) + gemcitabine more than tripled ORR vs gemcitabine alone
 CLOSE THE TAB
CLOSE THE TAB
ABRAXANE + GEMCITABINE
SIGNIFICANTLY IMPROVED PFSa
49% increase in median PFS with ABRAXANE (125 mg/m2 QW3/4) + gemcitabine compared with gemcitabine alone3
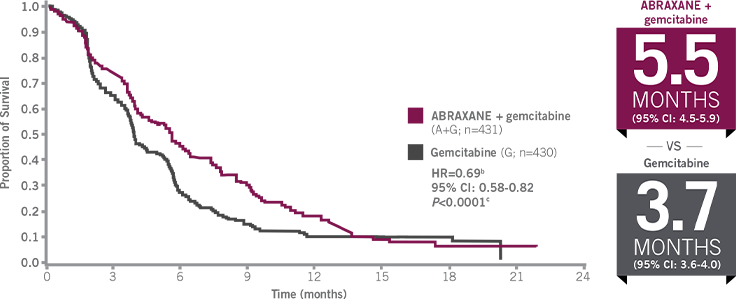
Based on independent radiological reviewer assessment.
Stratified using Cox proportional hazard model.
Based on a stratified log-rank test (stratified by geographic region, KPS, and presence of liver metastasis).
CLOSE THE TAB
WATCH
Dr. Kim Discusses the Largest Multinational Phase III Study

DOSING THAT DELIVERS
A well-established regimen for your patients
- Starting dose of 125 mg/m2
- Administer ABRAXANE, followed by gemcitabine 1000 mg/m2, on Days 1, 8, and 15 of each 28-day cycle
- 30- to 40-minute IV infusion
NOTE: DO NOT SUBSTITUTE FOR OR WITH OTHER PACLITAXEL FORMULATIONS.
ABRAXANE has different dosage and administration instructions from other paclitaxel products.
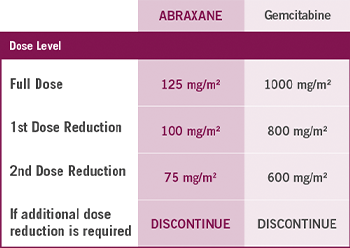
A WELL-DEFINED DOSE MODIFICATION SCHEDULE
Dose level reductions for patients with mPC
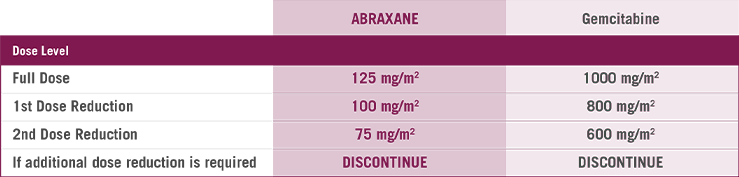

In the MPACT study4
- 41% of patients had at least one ABRAXANE dose reduction
- 71% of patients had at least one ABRAXANE dose withheld or delayed
STARTING DOSE FOR PATIENTS
WITH HEPATIC IMPAIRMENT

- Patients with bilirubin levels above the ULN were excluded from the MPACT study (central lab ULN in the MPACT study was 1.5 mg/dL)
AST=aspartate aminotransferase; SGOT=serum glutamic-oxaloacetic transaminase.
CLOSE THE TABUNDERSTANDING TIMING OF DOSE MODIFICATIONS
DURING ABRAXANE + GEMCITABINE THERAPY9
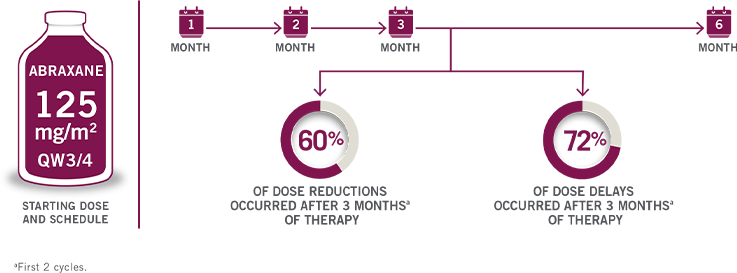
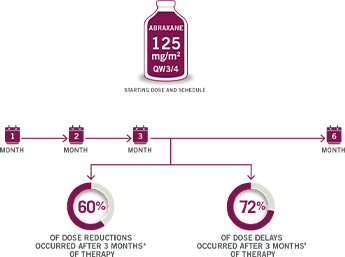
First 2 cycles.
CLOSE THE TABTHE APPROVED STARTING DOSE OF 125 mg/m2
Percentage of ABRAXANE patients receiving 125 mg/m2 at the start of each treatment cycle9,a
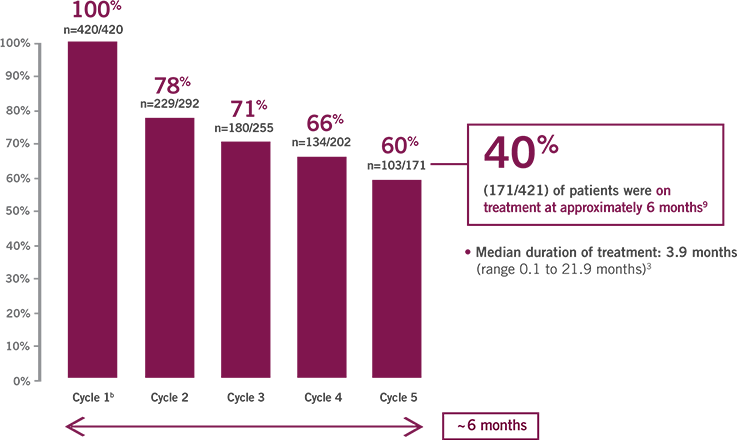
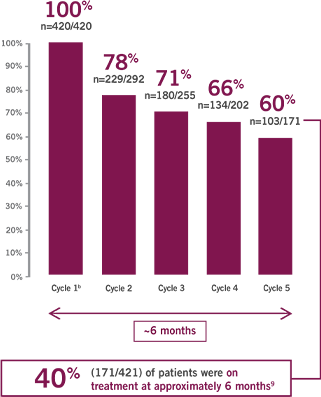
- Median duration of treatment: 3.9 months (range 0.1 to 21.9 months)3
Within the MPACT study there were also dose modifications to the gemcitabine dose within the ABRAXANE + gemcitabine arm. Those modifications are not shown here.
Cycle 1=8 weeks; subsequent cycles=4 weeks each.
CLOSE THE TABVIEW
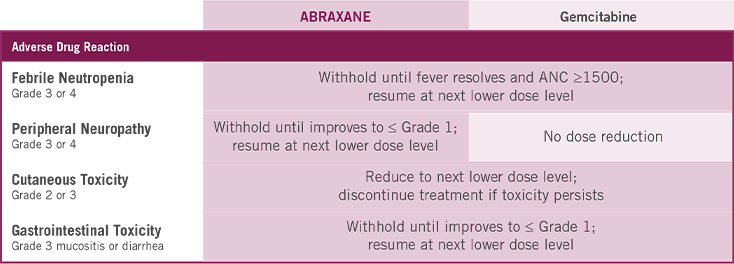
Dose Modifications for Severe Hematologic, Neurologic, Cutaneous, or Gastrointestinal Toxicity


DOWNLOAD NOW
Quick-reference Card for Dosing Schedule and Dose Modifications


WATCH
Dr. Kim Presents the Dosing and Schedule Recommendation for
ABRAXANE + Gemcitabine

A WELL-ESTABLISHED SAFETY PROFILE
Most common ARs (≥20%) with a ≥5% higher incidence for ABRAXANE + gemcitabine arm
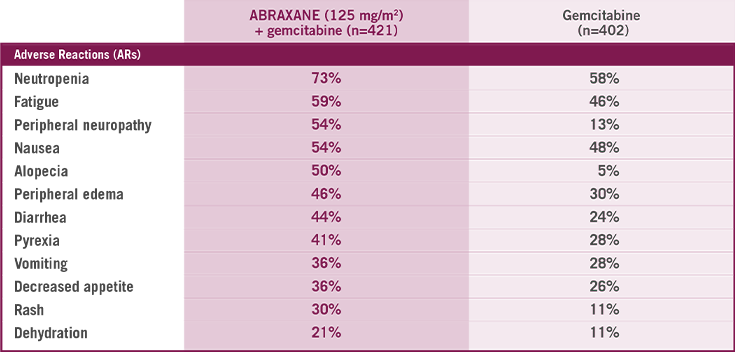
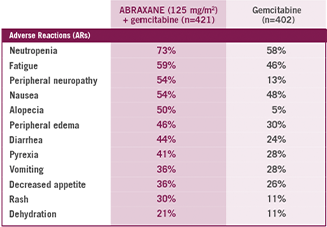
- ABRAXANE + gemcitabine had the same incidence of death due to adverse reactions compared to gemcitabine alone (4% of patients in each arm)4
- Disease progression was the most common reason for treatment discontinuation4
- The most common ARs resulting in permanent discontinuation of ABRAXANE were:
8% peripheral neuropathy | 4% fatigue | 2% thrombocytopenia
A WELL-ESTABLISHED SAFETY PROFILE
Higher incidence (≥5% for all grade toxicity or ≥2% Grade 3 or higher toxicity) in the ABRAXANE + gemcitabine arm
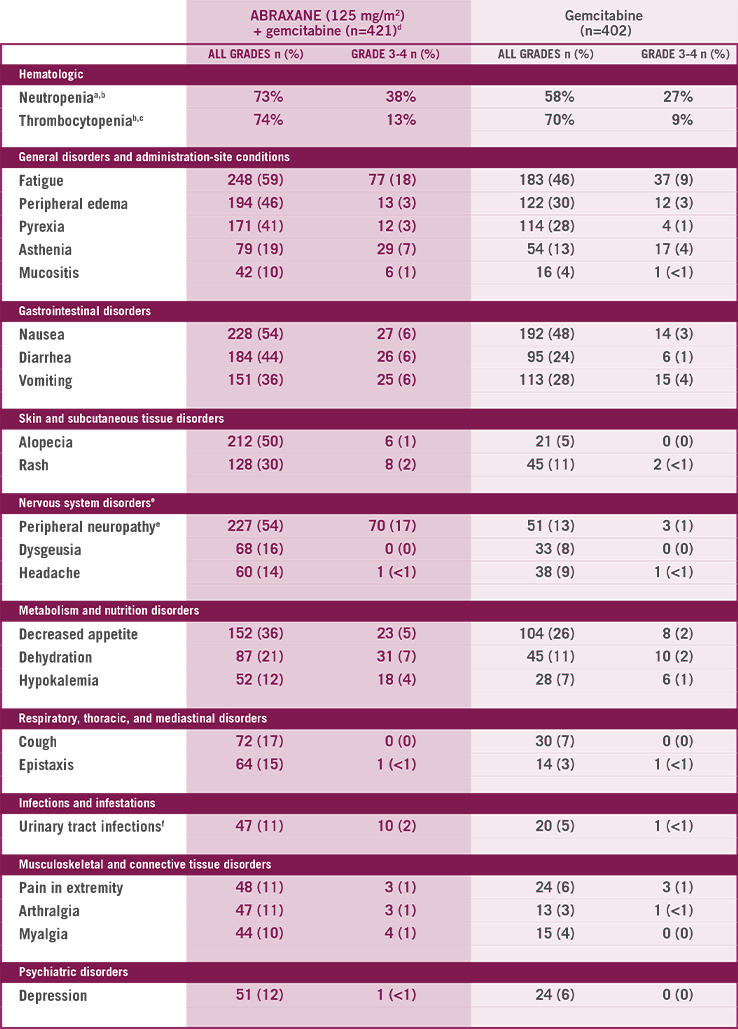
MedDRA=Medical Dictionary for Regulatory Activities.
405 patients assessed in ABRAXANE + gemcitabine–treated group.
388 patients assessed in gemcitabine-treated group.
404 patients assessed in ABRAXANE + gemcitabine–treated group.
Neutrophil growth factors were administered to 26% of patients in the ABRAXANE + gemcitabine group.
Peripheral neuropathy is defined by the MedDRA v15.0 Standardized MedDRA Query (broad scope).
Urinary tract infections includes the preferred terms of: urinary tract infection, cystitis, urosepsis, urinary tract infection bacterial, and urinary tract infection enterococcal.
A WELL-ESTABLISHED SAFETY PROFILE
Most common serious ARs (with a ≥1% higher incidence) for ABRAXANE + gemcitabine arm
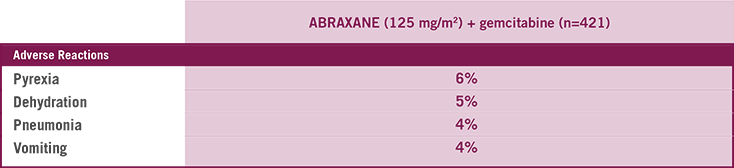
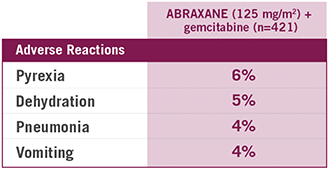 CLOSE THE TAB
CLOSE THE TAB

DOWNLOAD NOW
Quick-reference Card for Dosing Schedule and Dose Modifications


WATCH
Dr. Kim Reviews the Well-Established Safety Profile of ABRAXANE Based on the Phase III Study

PERIPHERAL NEUROPATHY IN THE MPACT STUDY

- 54% of patients experienced peripheral neuropathy of any grade (227/421)
- In the 17% of patients who experienced Grade 3 peripheral neuropathy (70/421), 44% (n=31) resumed ABRAXANE at a reduced dose
- The median time to first occurrence of Grade 3 peripheral neuropathy in the ABRAXANE arm was 140 days
- The median time to improvement from Grade 3 peripheral neuropathy to ≤ Grade 1 was 29 days after withholding dose
- 8% of patients who received ABRAXANE + gemcitabine permanently discontinued ABRAXANE due to peripheral neuropathy
APPROPRIATE DOSE MODIFICATIONS FOR PERIPHERAL NEUROPATHY
Assessing the severity of peripheral neuropathy is key to determining dose modifications




UNDERSTANDING HEMATOLOGIC ARs
WITH ABRAXANE + GEMCITABINE
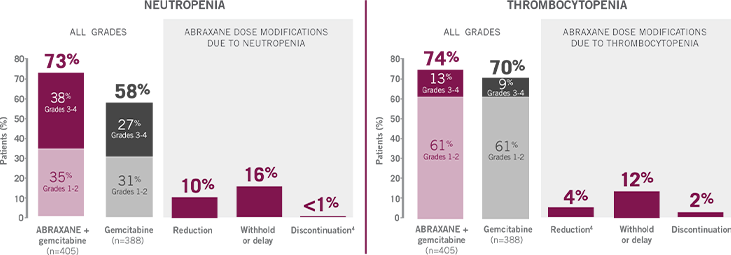
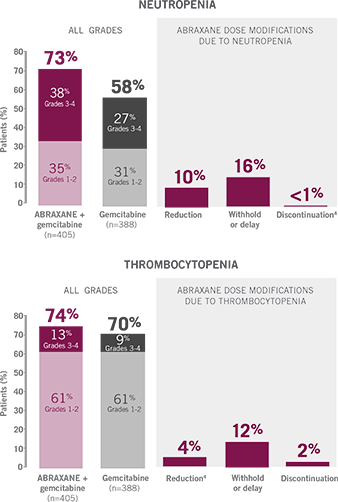
26% of ABRAXANE + gemcitabine patients received granulocyte colony stimulating factor (G-CSF).
APPROPRIATE DOSE MODIFICATIONS FOR HEMATOLOGIC ARs
Monitor for myelotoxicity by performing complete blood cell counts frequently, including prior to dosing on Days 1, 8, and 15

Assess whether to begin or delay treatment cycle (based on CBC prior to dosing on Day 1)
- Patients must have ANC ≥1500 cells/mm3 and platelet counts ≥100,000 cells/mm3 to initiate a cycle
- If counts are not at these levels, delay the start of the cycle until recovery
The recommended starting dose of ABRAXANE is 125 mg/m2
Adjust treatment as necessary within the cycle based on ANC and platelet counts
(determined via CBC prior to dosing on Days 8 and 15)



Treatment adjustments at Day 15 depend upon action taken at Day 8
SELECT ACTION TAKEN ON DAY 8 ABOVE TO SEE APPROPRIATE DOSE MODIFICATIONS FOR DAY 15
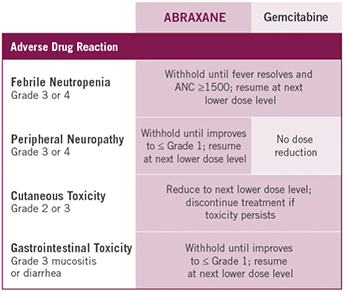
If Grade 3 or 4 febrile neutropenia occurs, withhold ABRAXANE and gemcitabine until fever resolves and ANC ≥1500; resume at next lower dose level.
CBC=complete blood count.
CLOSE THE TAB
DOWNLOAD NOW
Quick-reference Dosing Card, including Dose Modifications for Hematologic ARs