This website is best viewed using the horizontal display on your tablet device.
- INDICATIONS

ABRAXANE® is indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated.
ABRAXANE® is indicated for the first-line treatment of locally advanced or metastatic non–small cell lung cancer, in combination with carboplatin, in patients who are not candidates for curative surgery or radiation therapy.
ABRAXANE® is indicated for the first-line treatment of patients with metastatic adenocarcinoma of the pancreas, in combination with gemcitabine.
- FULL PRESCRIBING INFORMATION INCLUDING BOXED WARNING
- PATIENT INFORMATION
- PATIENT SITES
- BMS ACCESS SUPPORT
- BMS RESOURCES
THIS SITE IS INTENDED FOR U.S. HEALTHCARE PROFESSIONALS ONLY.

This website is best viewed using the vertical display on your mobile device.
THIS SITE IS INTENDED FOR U.S HEALTHCARE PROFESSIONALS ONLY.
PATIENTS WITH ADVANCED SQUAMOUS NSCLC ARE IN URGENT NEED OF OPTIONS THAT MAY DELIVER TUMOR RESPONSE3
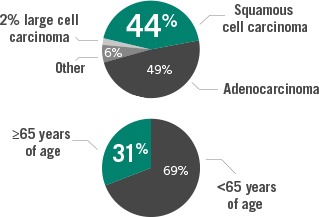
Lung cancer histology4
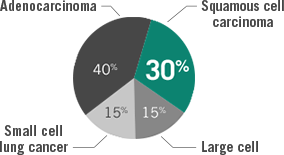
~30% of lung cancers are
squamous cell carcinoma
NSCLC=non–small cell lung cancer.
Squamous cell carcinomas
- Associated with a poorer prognosis than nonsquamous3
- Tumors are often centrally located5
- Have been shown to grow twice as fast as adenocarcinoma6
- Often seen extrinsically pushing into airway structures as they grow7
- Associated with a history of smoking5
NSCLC=non–small cell lung cancer.
RESPONSE RATES ARE HELPFUL IN ASSESSING CLINICAL BENEFIT
ORR is a clinically meaningful endpoint in NSCLC trials, which can help physicians choose an appropriate treatment8
Using ORR as an endpoint in oncology drug approval has a long history
Response criteria have been used for more than 30 years9
ORR=overall response rate.
TUMOR RESPONSE IS A RIGOROUS MEASUREMENT8
Spatial measurement of tumor shrinkage is a form of direct, real-time clinical assessment11
MEASURE OF THE GREATER UNIDIMENSIONAL DIAMETERS
RECIST Criteria10
CR=complete response
PR=partial response
SD=stable disease
PD=progressive disease
Response assessment under RECIST guidelines is based on measurement of the longest single-dimension diameters of target lesions, which are identified at baseline.
RECIST=Response Evaluation Criteria In Solid Tumors.
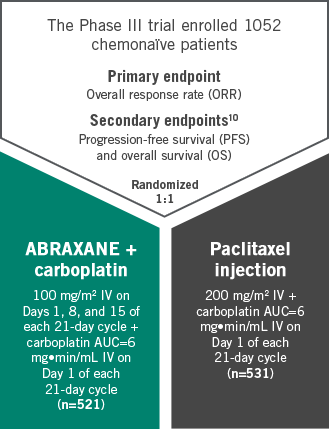
CLOSE THE TABTHE ABRAXANE PIVOTAL PHASE III TRIAL INCLUDED OVER 1000 PATIENTS WITH ADVANCED SQUAMOUS AND NONSQUAMOUS NSCLC
Treatment administered until disease progression or development of unacceptable toxicity
Studied in a broad range of chemonaïve patients with advanced NSCLC
Stratified by histologies and age10,11
ABRAXANE + carboplatin arm (n=521)
Three-quarters of the patients had a history of
smoking11
ABRAXANE + carboplatin arm (n=519)a


The smoking status was unknown for 2 patients in the ABRAXANE + carboplatin arm.
aNSCLC=advanced non–small cell lung cancer; AUC=area under the curve; IV=intravenously.

See ORR for ABRAXANE + carboplatin vs paclitaxel in aNSCLC

ABRAXANE + CARBOPLATIN DELIVERED SIGNIFICANTLY
SUPERIOR ORR VS PACLITAXEL
ORR in the ITT population of the Phase III trial for first-line advanced or metastatic NSCLC
![Overall response rate in the ITT population was 33% with ABRAXANE + carboplatin (n=170/521 [95% CI: 28.6%-36.7%]) vs 25% with paclitaxel injection + carboplatin (n=132/531 [95% CI: 21.2%-28.5%]) (P=0.005) - bar chart](/assets/commercial/us/abraxanepro/en/images/metaNSCLC_07.png)
![Overall response rate in the ITT population was 33% with ABRAXANE + carboplatin (n=170/521 [95% CI: 28.6%-36.7%]) vs 25% with paclitaxel injection + carboplatin (n=132/531 [95% CI: 21.2%-28.5%]) (P=0.005) - bar chart](/assets/commercial/us/abraxanepro/en/images/metaNSCLC_07_mob.png)
P=0.005 (based on chi-square test).
- Median duration of response was 6.9 months vs 6.0 months (95% CI: 5.6-8.0 vs 5.6-7.1, respectively)
- No statistically significant difference in OS between the 2 study arms (median OS 12.1 months vs 11.2 months, P=NS10)
ITT=intent-to-treat; NS=not significant.
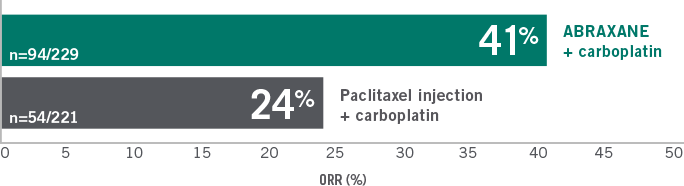
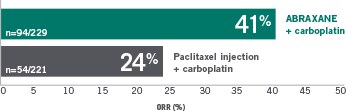
41% ORR IN FIRST-LINE SQUAMOUS
Squamous advanced or metastatic NSCLC
RESPONSE RATE IN OTHER HISTOLOGIES
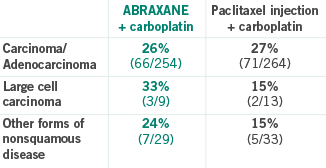
Carcinoma/Adenocarcinoma
- 26% (66/254) for ABRAXANE + carboplatin vs 27% (71/264) for paclitaxel injection + carboplatin
Large cell carcinoma
- 33% (3/9) for ABRAXANE + carboplatin vs 15% (2/13) for paclitaxel injection + carboplatin
Other forms of nonsquamous disease
- 24% (7/29) for ABRAXANE + carboplatin vs 15% (5/33) for paclitaxel injection + carboplatin
WELL-ESTABLISHED SAFETY PROFILE
Selected ARs with a difference of ≥5% for all grades or ≥2% for Grades 3-4 toxicity between treatment groups

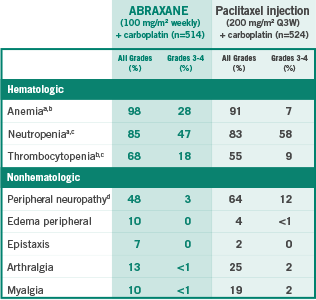
508 patients assessed in ABRAXANE + carboplatin–treated group.
514 patients assessed in paclitaxel injection + carboplatin–treated group.
513 patients assessed in paclitaxel injection + carboplatin–treated group.
Peripheral neuropathy is defined by the MedDRA v14.0 SMQ neuropathy (broad scope).
AR=adverse reaction; MedDRA=Medical Dictionary for Regulatory Activities; Q3W=every 3 weeks; SMQ=Standardized MedDRA Query.
ESTABLISHED SAFETY PROFILE IN ADVANCED NSCLC
Common ARs (≥10%) for ABRAXANE + carboplatin observed at a similar incidence as with paclitaxel injection + carboplatin

 CLOSE THE TAB
CLOSE THE TAB

Download the “Preparing for My Treatment” Brochure: Help Your Patients With
Advanced NSCLC Get Ready for Their Medical Appointments

GRADE 3 NEUROPATHY: MEDIAN TIME TO IMPROVEMENT WITHIN
1 MONTH AFTER DOSE
MODIFICATION11
48% of patients who received ABRAXANE + carboplatin developed any grade peripheral neuropathy compared with 64% of patients in the paclitaxel + carboplatin arm
Grade 3 peripheral neuropathy in the ABRAXANE + carboplatin arm vs paclitaxel + carboplatin
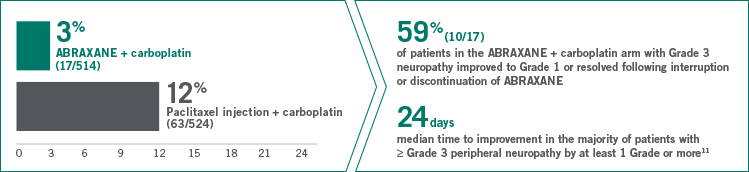
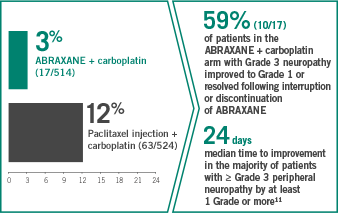
- 1% of patients discontinued ABRAXANE due to peripheral neuropathy
- No patients in the ABRAXANE + carboplatin arm developed Grade 4 peripheral neuropathy

Learn About Dose Modification for Treatment-related Neuropathy

DOSAGE & ADMINISTRATION: ABRAXANE + CARBOPLATIN


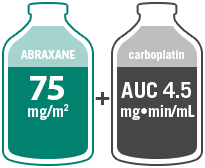
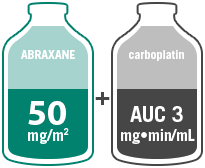
- Administer ABRAXANE intravenously at a dose of 100 mg/m2 over 30 minutes on a weekly schedule on Days 1, 8, and 15 of each 21-day cycle
- Administer carboplatin intravenously on Day 1 of each 21-day cycle immediately after completion of ABRAXANE administration
Note: DO NOT SUBSTITUTE FOR OR WITH OTHER PACLITAXEL FORMULATIONS. ABRAXANE has different dosage and administration instructions from other paclitaxel products.
ABRAXANE is administered generally without the need for steroid pretreatment
- Premedication may be needed in patients who have had a prior hypersensitivity reaction to ABRAXANE
- Severe hypersensitivity reactions with fatal outcome have been reported with ABRAXANE. Patients who experience a severe hypersensitivity reaction to ABRAXANE should not be rechallenged with this drug
- Patients with a previous history of hypersensitivity to other taxanes should be closely monitored during initiation of ABRAXANE therapy
MEDIAN NUMBER OF TREATMENT CYCLES IN THE PHASE III PIVOTAL TRIAL OF ABRAXANE + CARBOPLATIN


Patients in the study were treated until disease progression or development of unacceptable toxicity.
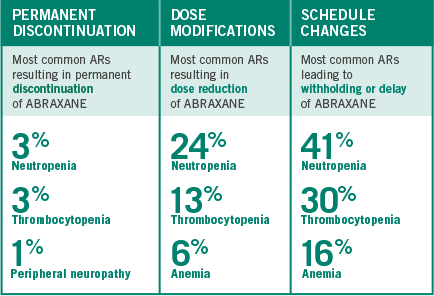
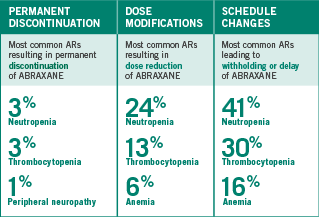
DOSE ADJUSTMENTS OBSERVED IN THE PHASE III PIVOTAL TRIAL
Adverse reactions were assessed in 514 patients treated with ABRAXANE + carboplatin
PLEASE SELECT A DOSE MODIFICATION BELOW:
Recommendations for starting dose in patients with hepatic impairment
SGOT (AST) LEVELS
<10 x ULN
AND
BILIRUBIN LEVELS
> ULN TO ≤1.5 x ULN
SGOT (AST) LEVELS
<10 x ULN
AND
BILIRUBIN LEVELS
> 1.5 TO ≤3 x ULN
SGOT (AST) LEVELS
<10 x ULN
AND
BILIRUBIN LEVELS
> 3 TO ≤5 x ULN
SGOT (AST) LEVELS
>10 x ULN
AND
BILIRUBIN LEVELS
>5 x ULN
Mild
- No dose adjustment is necessary for patients with mild hepatic impairment
- Do not administer ABRAXANE to patients with total bilirubin >5 x ULN or AST >10 x ULN
- Patients with bilirubin levels above the ULN were excluded from the clinical trial for lung cancer
- Dosage recommendations are for the first course of therapy. The need for further dose adjustments in subsequent courses should be based on individual tolerance
AST=aspartate aminotransferase; SGOT=serum glutamic-oxaloacetic transaminase; ULN=upper limit of normal.
Moderate
- Do not administer ABRAXANE to patients with total bilirubin >5 x ULN or AST >10 x ULN
- Patients with bilirubin levels above the ULN were excluded from the clinical trial for lung cancer
- Dosage recommendations are for the first course of therapy. The need for further dose adjustments in subsequent courses should be based on individual tolerance
AST=aspartate aminotransferase; SGOT=serum glutamic-oxaloacetic transaminase; ULN=upper limit of normal.
Severe
- Do not administer ABRAXANE to patients with total bilirubin >5 x ULN or AST >10 x ULN
- Patients with bilirubin levels above the ULN were excluded from the clinical trial for lung cancer
- Dosage recommendations are for the first course of therapy. The need for further dose adjustments in subsequent courses should be based on individual tolerance
AST=aspartate aminotransferase; SGOT=serum glutamic-oxaloacetic transaminase; ULN=upper limit of normal.
Very Severe
- Do not administer ABRAXANE to patients with total bilirubin >5 x ULN or AST >10 x ULN
- Patients with bilirubin levels above the ULN were excluded from the clinical trial for lung cancer
AST=aspartate aminotransferase; SGOT=serum glutamic-oxaloacetic transaminase; ULN=upper limit of normal.








ILLUSTRATIVE PURPOSES ONLY
A dose increase to 100 mg/m2 in subsequent courses should be considered if the patient tolerates the reduced dose for 2 cycles.
Recommendations for permanent dose modification in NSCLC patients with neutropenia
MAKE A SELECTION
- Do not administer ABRAXANE on Day 1 of a cycle until absolute neutrophil count
(ANC) is at least 1500 cells/mm3 and platelet count is at least 100,000 cells/mm3 - Nadir ANC <500 cells/mm3 with neutropenic fever >38 °C
- Delay of next cycle due to persistent neutropeniaa (nadir ANC <1500 cells/mm3)
- Nadir ANC <500 cells/mm3 for >1 week
- Prior to permanent dose reduction, withhold treatment until ANC recovery to ≥1500 cells/mm3 on Day 1 or ≥500 cells/mm3 on Day 8 or 15 of the cycle
- Monitor for myelotoxicity by performing complete blood cell counts frequently, including prior to dosing on Days 1, 8, and 15
- In the Phase III ABRAXANE NSCLC clinical trial, 85% of patients treated with ABRAXANE (100 mg/m2 weekly) + carboplatin experienced Grades 1-4 neutropenia; 47% experienced Grades 3-4 neutropenia
OR
OR
More than 7-day delay of scheduled Day 1 dose of next cycle.
- Do not administer ABRAXANE on Day 1 of a cycle until absolute neutrophil count
(ANC) is at least 1500 cells/mm3 and platelet count is at least 100,000 cells/mm3 - Nadir ANC <500 cells/mm3 with neutropenic fever >38 °C
- Delay of next cycle due to persistent neutropeniaa (nadir ANC <1500 cells/mm3)
- Nadir ANC <500 cells/mm3 for >1 week
- Prior to permanent dose reduction, withhold treatment until ANC recovery to ≥1500 cells/mm3 on Day 1 or ≥500 cells/mm3 on Day 8 or 15 of the cycle
- Monitor for myelotoxicity by performing complete blood cell counts frequently, including prior to dosing on Days 1, 8, and 15
- In the Phase III ABRAXANE NSCLC clinical trial, 85% of patients treated with ABRAXANE (100 mg/m2 weekly) + carboplatin experienced Grades 1-4 neutropenia; 47% experienced Grades 3-4 neutropenia
OR
OR
More than 7-day delay of scheduled Day 1 dose of next cycle.
- Do not administer ABRAXANE on Day 1 of a cycle until absolute neutrophil count
(ANC) is at least 1500 cells/mm3 and platelet count is at least 100,000 cells/mm3 - Nadir ANC <500 cells/mm3 with neutropenic fever >38 °C
- Delay of next cycle due to persistent neutropeniaa (nadir ANC <1500 cells/mm3)
- Nadir ANC <500 cells/mm3 for >1 week
- Prior to permanent dose reduction, withhold treatment until ANC recovery to ≥1500 cells/mm3 on Day 1 or ≥500 cells/mm3 on Day 8 or 15 of the cycle
- Monitor for myelotoxicity by performing complete blood cell counts frequently, including prior to dosing on Days 1, 8, and 15
- In the Phase III ABRAXANE NSCLC clinical trial, 85% of patients treated with ABRAXANE (100 mg/m2 weekly) + carboplatin experienced Grades 1-4 neutropenia; 47% experienced Grades 3-4 neutropenia
OR
OR
More than 7-day delay of scheduled Day 1 dose of next cycle.



ILLUSTRATIVE PURPOSES ONLY
Recommendations for permanent dose modification in NSCLC patients with thrombocytopenia
MAKE A SELECTION
- Do not administer ABRAXANE on Day 1 of a cycle until absolute neutrophil count
(ANC) is at least 1500 cells/mm3 and platelet count is at least 100,000 cells/mm3 - Prior to permanent dose reduction, withhold treatment until platelet count
recovery to ≥100,000 cells/mm3 on Day 1 or ≥50,000 cells/mm3 on Days 8
or 15 of the cycle - Monitor for myelotoxicity by performing complete blood cell counts frequently,
including prior to dosing on Days 1, 8, and 15 - In the Phase III ABRAXANE NSCLC clinical trial, 68% of patients treated with ABRAXANE (100 mg/m2 weekly)
+ carboplatin experienced Grades 1-4 thrombocytopenia; 18% experienced Grades 3-4 thrombocytopenia
- Do not administer ABRAXANE on Day 1 of a cycle until absolute neutrophil count
(ANC) is at least 1500 cells/mm3 and platelet count is at least 100,000 cells/mm3 - Prior to permanent dose reduction, withhold treatment until platelet count
recovery to ≥100,000 cells/mm3 on Day 1 or ≥50,000 cells/mm3 on Days 8
or 15 of the cycle - Monitor for myelotoxicity by performing complete blood cell counts frequently,
including prior to dosing on Days 1, 8, and 15 - In the Phase III ABRAXANE NSCLC clinical trial, 68% of patients treated with ABRAXANE (100 mg/m2 weekly)
+ carboplatin experienced Grades 1-4 thrombocytopenia; 18% experienced Grades 3-4 thrombocytopenia
Nadir platelets <50,000 cells/mm3




ILLUSTRATIVE PURPOSES ONLY
Recommendations for permanent dose modification in NSCLC patients with neurologic toxicity
MAKE A SELECTION
- For Grades 3-4 peripheral neuropathy, withhold treatment until resolution to ≤ Grade 1
- Treatment may be resumed at the next lower dose level in subsequent cycles according to the dose reduction guidelines for neurologic toxicity
- In the Phase III ABRAXANE NSCLC clinical trial, 48% of patients treated with ABRAXANE (100 mg/m2 weekly) + carboplatin experienced Grades 1-4 peripheral neuropathy; 3% experienced Grades 3-4 peripheral neuropathy; no Grade 4 peripheral neuropathy was seen in the ABRAXANE + carboplatin arm
- For Grades 3-4 peripheral neuropathy, withhold treatment until resolution to ≤ Grade 1
- Treatment may be resumed at the next lower dose level in subsequent cycles according to the dose reduction guidelines for neurologic toxicity
- In the Phase III ABRAXANE NSCLC clinical trial, 48% of patients treated with ABRAXANE (100 mg/m2 weekly) + carboplatin experienced Grades 1-4 peripheral neuropathy; 3% experienced Grades 3-4 peripheral neuropathy; no Grade 4 peripheral neuropathy was seen in the ABRAXANE + carboplatin arm
- For Grades 3-4 peripheral neuropathy, withhold treatment until resolution to ≤ Grade 1
- Treatment may be resumed at the next lower dose level in subsequent cycles according to the dose reduction guidelines for neurologic toxicity
- In the Phase III ABRAXANE NSCLC clinical trial, 48% of patients treated with ABRAXANE (100 mg/m2 weekly) + carboplatin experienced Grades 1-4 peripheral neuropathy; 3% experienced Grades 3-4 peripheral neuropathy; no Grade 4 peripheral neuropathy was seen in the ABRAXANE + carboplatin arm
Severe sensory neuropathy
(Grade 3 or 4)






ILLUSTRATIVE PURPOSES ONLY

Watch the ABRAXANE Reconstitution Video

ADVANCED OR METASTATIC NON–SMALL CELL
LUNG CANCER (mNSCLC)
NCCN CLINICAL PRACTICE GUIDELINES IN ONCOLOGY
(NCCN GUIDELINES®) RECOMMENDATIONS12*
Initial Systemic Therapy
All Histologies Advanced or Metastatic Non–Small Cell Lung Cancer (mNSCLC)
Albumin-bound paclitaxel (ABRAXANE) + carboplatin
NCCN
Category 1a
Albumin-bound paclitaxel (ABRAXANE) + carboplatin is a treatment option included in the NCCN Guidelines® for Non-Small Cell Lung Cancer with a Category 1 recommendation for the first-line treatment of metastatic squamous cell carcinoma and nonsquamous NSCLC (PS 0-1)b
For additional considerations and treatment options, see NCCN.org.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
EGFR, ALK, ROS1, BRAF, MET exon 14 skipping mutation, and RET negative, treatment option for patients with contraindications to PD-1 or PD-L1 inhibitors. Contraindications for treatment with PD-1/PD-L1 inhibitors may include active or previously documented autoimmune disease and/or current use of immunosuppressive agents or presence of an oncogene, which would predict lack of benefit.
ALK=anaplastic lymphoma kinase; BRAF=B-Raf proto-oncogene; EGFR=epidermal growth factor receptor; MET=mesenchymal-epithelial transition; NCCN=National Comprehensive Cancer Network; PD-1=programmed cell death protein 1; PD-L1=programmed death-ligand 1; PS=performance status; RET=rearranged during transfection; ROS1=c-ros oncogene 1.

See how ABRAXANE + carboplatin delivered a significantly superior ORR
vs paclitaxel in mNSCLC

ABRAXANE + CARBOPLATIN FOR FIRST-LINE ADVANCED OR
METASTATIC NSCLC, INCLUDING SQUAMOUS
ABRAXANE: Pivotal Phase III study
ABRAXANE + carboplatin delivered significantly superior ORR vs paclitaxel: ITT population10
ABRAXANE + carboplatin
(n=170/521); 95% CI: 28.6%-36.7%
Paclitaxel injection + carboplatin
(n=132/531); 95% CI: 21.2%-28.5%
- Median duration of response was 6.9 months vs 6.0 months (95% CI: 5.6-8.0 vs 5.6-7.1, respectively)
- No statistically significant difference in OS between the 2 study arms (median OS 12.1 months vs 11.2 months, P=NS10)
P=0.005 (based on chi-square test).
41% ORR in first-line squamous:
Squamous population
ABRAXANE + carboplatin
(n=94/229)
Paclitaxel injection + carboplatin
(n=54/221)
Response rate in other histologies

Established safety profile
The most common ARs (≥20%) of ABRAXANE in combination with carboplatin are anemia, neutropenia, thrombocytopenia, alopecia, peripheral neuropathy, nausea, and fatigue.
NCCN Guidelines Recommendations12*
Initial Systemic Therapy
All Histologies
Advanced or Metastatic NSCLC
Albumin-bound paclitaxel
(ABRAXANE) + carboplatin
NCCN Category 1†
Albumin-bound paclitaxel (ABRAXANE) + carboplatin is a treatment option included in the NCCN Guidelines® for Non-Small Cell Lung Cancer with a Category 1 recommendation for the first-line treatment of metastatic squamous cell carcinoma and nonsquamous NSCLC (PS 0-1)‡
For additional considerations and treatment options, see NCCN.org.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
EGFR, ALK, ROS1, BRAF, MET exon 14 skipping mutation, and RET negative, treatment option for patients with contraindications to PD-1 or PD-L1 inhibitors. Contraindications for treatment with PD-1/PD-L1 inhibitors may include active or previously documented autoimmune disease and/or current use of immunosuppressive agents or presence of an oncogene, which would predict lack of benefit.
Study design
Multicenter, randomized 1:1, Phase III study comparing ABRAXANE (100 mg/m2 IV; Days 1, 8, and 15 of each 21-day cycle) + carboplatin (AUC=6 mg•min/mL IV, Day 1 of each 21-day cycle) with paclitaxel injection (200 mg/m2 IV, Day 1 of each 21-day cycle) + carboplatin (AUC=6 mg•min/mL IV, Day 1 of each 21-day cycle) in 1052 chemonaïve patients with advanced NSCLC. The primary efficacy endpoint was ORR.10

Access Resources for Both You and Your Advanced NSCLC Patients