This website is best viewed using the horizontal display on your tablet device.
- INDICATIONS

ABRAXANE® is indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated.
ABRAXANE® is indicated for the first-line treatment of locally advanced or metastatic non–small cell lung cancer, in combination with carboplatin, in patients who are not candidates for curative surgery or radiation therapy.
ABRAXANE® is indicated for the first-line treatment of patients with metastatic adenocarcinoma of the pancreas, in combination with gemcitabine.
- FULL PRESCRIBING INFORMATION INCLUDING BOXED WARNING
- PATIENT INFORMATION
- PATIENT SITES
- BMS ACCESS SUPPORT
- BMS RESOURCES
THIS SITE IS INTENDED FOR U.S. HEALTHCARE PROFESSIONALS ONLY.

This website is best viewed using the vertical display on your mobile device.
THIS SITE IS INTENDED FOR U.S HEALTHCARE PROFESSIONALS ONLY.
- ABOUT ABRAXANE
- Overview
- Product Attributes
- Dosing Summary
- Pharmacokinetics
DELIVER THE DIFFERENCE OF AN
ALBUMIN-BOUND PACLITAXEL
ABRAXANE is an albumin-bound nanoparticle formulation
- ABRAXANE is a microtubule inhibitor
- ABRAXANE contains albumin (human), a derivative of human blood, and has a mean particle size of approximately 130 nanometers
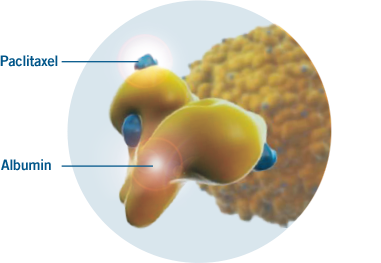
VISUAL REPRESENTATION
BASED ON
PRECLINICAL EVIDENCE.
PRODUCT ATTRIBUTES
NOT ACTUAL SIZE.
- ABRAXANE is formulated with albumin and is solvent-free
- Premedication for hypersensitivity is generally not required
- Premedication may be needed in patients who had prior hypersensitivity reactions to ABRAXANE
- ABRAXANE can be infused over 30-40 minutes. Exact administration times and dosage vary across indications
FDA-APPROVED DOSE AND SCHEDULE
Metastatic
Pancreatic Cancer (mPC)

ILLUSTRATIVE PURPOSES ONLY.
- Administer ABRAXANE followed by gemcitabine weekly for 3 weeks, then 1 week break of each 28-day cycle
- ABRAXANE is administered intravenously over 30-40 minutes
- ABRAXANE is indicated for the first-line treatment of patients with metastatic adenocarcinoma of the pancreas, in combination with gemcitabine
Metastatic
Breast Cancer (MBC)

ILLUSTRATIVE PURPOSES ONLY.
- Administer ABRAXANE once every 3 weeks
- ABRAXANE is administered intravenously over 30 minutes
- ABRAXANE is indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated
Advanced Non–Small
Cell Lung Cancer (NSCLC)

ILLUSTRATIVE PURPOSES ONLY.
- Administer ABRAXANE on Days 1, 8, and 15 of each 21-day cycle
- Administer carboplatin on Day 1 only of each 21-day cycle, beginning immediately after the completion of ABRAXANE administration
- ABRAXANE is administered intravenously over 30 minutes
- ABRAXANE is indicated for the first-line treatment of locally advanced or metastatic non–small cell lung cancer, in combination with carboplatin, in patients who are not candidates for curative surgery or radiation therapy
PHARMACOKINETICS
The pharmacokinetics (PK) of total paclitaxel following 30- and 180-minute infusions of ABRAXANE at dose levels of 80 to 375 mg/m2 were determined in clinical studies.
- Following intravenous administration of ABRAXANE, paclitaxel plasma concentrations declined in a biphasic manner
- The initial rapid decline represents distribution to the peripheral compartment
- The slower second phase represented drug elimination
The drug exposure (AUCs) was dose proportional over 80 to 300 mg/m2, and the PK of paclitaxel for ABRAXANE were independent of the duration of intravenous administration.
PK of ABRAXANE (260 mg/m2 over 30 minutes) vs paclitaxel injection (175 mg/m2 over 3 hours)
- ABRAXANE clearance was 43% larger than paclitaxel injection
- ABRAXANE has a 53% higher volume of distribution than paclitaxel injection
Following ABRAXANE administration to patients with solid tumors, paclitaxel is evenly distributed into blood cells and plasma and is highly bound to plasma proteins (94%).
In a within-patient comparison study, the fraction of unbound paclitaxel in plasma was significantly higher with ABRAXANE (6.2%) than with solvent-based paclitaxel (2.3%).
- This contributes to significantly higher exposure to unbound paclitaxel with ABRAXANE compared with solvent-based paclitaxel, when the total exposure is comparable
In vitro studies demonstrating the binding qualities of ABRAXANE using paclitaxel concentrations ranging from 0.1 to 50 μg/mL indicate that
- The presence of cimetidine, ranitidine, dexamethasone, or diphenhydramine did not affect protein binding of paclitaxel
The total volume of distribution is approximately 1741 L
- The large volume of distribution indicates extensive extravascular distribution and/or tissue binding of paclitaxel
In vitro studies with human liver microsomes showed that paclitaxel was metabolized primarily to 6α-hydroxypaclitaxel by CYP2C8, and to two minor metabolites, 3’-p-hydroxypaclitaxel and 6α, 3’-p-dihydroxypaclitaxel, by CYP3A4.
In vitro, the metabolism of paclitaxel to 6α-hydroxypaclitaxel was inhibited by a number of agents
- Ketoconazole, verapamil, diazepam, quinidine, dexamethasone, cyclosporine, teniposide, etoposide, and vincristine
- Testosterone, 17α-ethinyl estradiol, retinoic acid, and quercetin, a specific inhibitor of CYP2C8
The PK of paclitaxel may also be altered in vivo as a result of interactions with compounds that are substrates, inducers, or inhibitors of CYP2C8 and/or CYP3A4.
At the clinical dose range of 80 to 300 mg/m2, the mean total clearance of paclitaxel ranges from 13 to 30 L/h/m2, and the mean terminal half-life ranges from 13 to 27 hours.
After a 30-minute infusion of 260 mg/m2 dose of ABRAXANE, the mean value for cumulative urinary recovery of unchanged drug (4%) indicated extensive non-renal clearance. Less than 1% of the total administered dose was excreted in urine as the metabolites 6α-hydroxypaclitaxel and 3'-p-hydroxypaclitaxel.
Fecal excretion was approximately 20% of the total dose administered.
